Silver Plating – The complete beginners guide
Silver plating is the coating of a metal surface with a layer of silver to improve appearance, conductivity and corrosion resistance. The widespread use of this technique spans from electronics to jewelry and this method has the benefits of excellent electrical conductivity, antibacterial properties and superior reflectivity. Electroplating or electroless silver plating assures a durable and high quality finish. The following guide will cover the basics of the techniques used, the applications and the benefits of silver plating so that the beginner can understand how it is done and why it is still used.
Table of Contents
ToggleHistorical Background of Silver Plating
The history of silver plating goes back as far as ancient Romans and Byzantines who used mercury amalgams and fire gilding. Modern silver plating started in the 19th century with pioneers such as Volta, Faraday and Berzelius, who pioneered electroplating techniques. Cyanide baths were widely used in the 19th and 20th centuries and recent advances include electroless plating and eco-friendly silver inks. Today, the silver plating is a blend of the traditional methods and modern, sustainable technologies.

Properties of Silver That Make It Ideal for Plating
Electrical Conductivity
For components requiring fast, efficient current flow, silver plating guarantees top performance. This is why it is widely used in electronics and power systems.
Thermal Conductivity
Silver gives you good heat dissipation. Because of its superior thermal conductivity, it is ideal for plating parts into high temperature environments. This also helps protect sensitive components from overheating.
Corrosion Resistance
While not entirely immune to corrosion, silver does well in certain environments. In moderately corrosive environments, you can count on silver plating to create a protective barrier that will help to prolong the life of your product.

Lubricity
Silver has a low coefficient of friction under the right conditions. This property reduces wear if you’re working with moving parts. Mechanical systems will be more efficient and durable.
Antibacterial Properties
Silver is a good metal for medical and food grade products due to its natural ability to combat bacteria. In hygiene critical applications, silver plating provides you with a cleaner, safer surface.
Reflectivity
Silver plating gives you excellent light reflection. It is therefore used in optical, mirror and solar energy devices. This improves brightness and increases energy efficiency.
Overview of Silver Plating Techniques
Electroplating
You run an external current to bring Ag⁺ ions onto your workpiece. It’s a silver or inert anode, your conductive item as cathode and a silver salt electrolyte. You get good thickness control and good adhesion using this method.
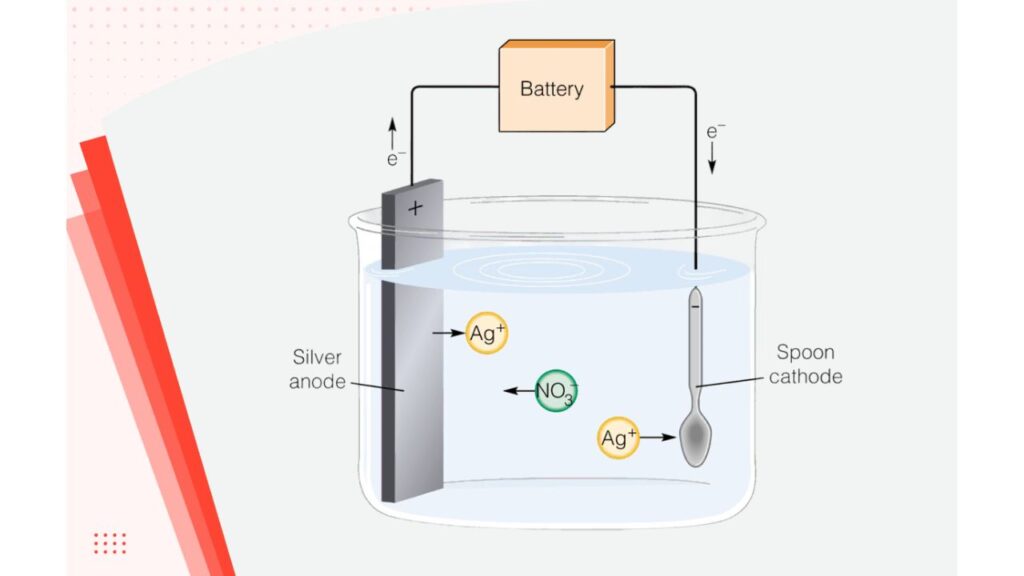
Chemical (or Electroless) Plating
There’s no electricity here. In this process, Ag⁺ ions are reduced with a chemical reaction. Formaldehyde or hydrazine are used as reducing agents and complexing agents keep the solution stable. When you want to coat non conductive surfaces is great.
Immersion (Displacement) plating
This is quick and easy. When you drop a more reactive metal such as copper, into a silver solution, it displaces silver ions and leaves a thin layer of silver. The coating is usually very thin, but you do get a decorative finish.
Specialized or Hybrid Methods
You might seek out more advanced techniques such as pulse reverse electroplating, plasma enhanced plating or vacuum deposition. They suit high precision or niche applications and provide tighter control of coating properties.
Pre-Treatment and Surface Preparation
Surface Cleaning
Degreasing
Begin by removing oils, greases and fingerprints. You can draw them in alkaline or solvent based baths. This step prepares your a clean, oil free surface for further treatment.
Ultrasonic Cleaning
Ultrasonic cleaning is best for delicate or complex parts. It employs cavitation bubbles to extract fine particles that can not be reached manually. This is ideal for precision components.

Acid or Alkaline Pickling
Pickling removes oxides, scale and rust. Sulfuric or hydrochloric are common acids. Chemically, this prepares the surface and improves the next treatment step.
Surface Activation

Acid Activation
Dip the part briefly in a dilute acid solution. This provides you with a hydrophilic surface which is better for your adhesion. It’s simple and very effective.
Catalyzation for Electroless
Catalyzation is a must if you’re plating on a plastic or a non conductive material. The surface is then made receptive to electroless plating by depositing a thin palladium or silver layer.
Mechanical Treatments

Abrasive Blasting or Polishing.
This can be used to vary surface roughness. Coatings stick better to a rougher surface. Select your media carefully according to material type and desired finish.
Etching
Etching provides micro-roughness which increases the surface area. This provides stronger mechanical bonding and keeps your coatings from peeling.
Rinsing and drying
Rinse thoroughly between steps. It avoids chemical cross contamination. Use deionized water for the final rinse for best results. Remove all of the moisture before finishing with air knife or oven drying.
Electroplating Process in Detail
Bath Composition (Electrolyte Chemistry)
As your metal source, you will need silver salts like silver cyanide (AgCN) or silver nitrate (AgNO₃). Thiosulfate or hydantoin based complexing agents are the way to go if you want a non cyanide setup. The quality of the deposit is improved with additives such as thiourea based brighteners, wetting agents, anti pitting agents and grain refiners.
Operating Parameters
Keep the bath temperature between 20 °C and 50 °C. Plating is accelerated by higher temperatures but grain structure is affected. If you use cyanide baths, maintain a pH of 9 to 10. Buffers stabilize the pH for you. To control thickness and finish, use a current density that corresponds to the surface area of your deposition—expressed in amps per square decimeter (A/dm²). Ensure ions spread evenly in the bath by adding air or mechanical agitation.
Anode and Cathode Selection
When you want the anodes to dissolve and add silver to the bath, use silver anodes. If not, inert anodes such as titanium mesh will be the best option. For the cathode, rack properly to distribute current evenly. It prevents you from having burn marks or low plate ‘shadow’ areas.
Deposition Control and Monitoring
A coulometric method or inline XRF tool can be used to measure the thickness of deposit accurately. Visually inspect the plating for color, brightness and defects, always. Maintain consistent logs of current, time, temperature and bath chemistry.
Electroless Silver Plating
Principles of Autocatalytic Plating
You reduce Ag⁺ ions to metallic silver using reducing agents like formaldehyde or sodium borohydride. It occurs with no external current applied. After the reaction begins on a catalytic surface, it continues uniformly across the object.
Bath Components and Chemistry
Starting with a silver salt like silver nitrate or silver oxide, you start. However, we add complexing agents (ammonia, citrate or thiosulfate) to keep Ag⁺ stable in solution. Reduction agents must be balanced carefully — too strong and silver may precipitate. Buffers maintain the pH slightly alkaline (10–11) and stabilizers such as surfactants or small lead salts, prevent unwanted reactions.
Activation of Substrate
If you are plating non conducting parts, you deposit a seed layer first. When starting, we sensitize the surface with SnCl₂ and then use PdCl₂ to form palladium nuclei. The ability of silver to properly anchor during plating is made possible by this layer.
Process Parameters
Bath temperatures are to be maintained between 35 °C and 50 °C. Plating is faster with higher heat but bath life may be shorter. Deposition is carried out gently stirring or air bubbling to avoid dead zones and ensure even deposition.
Controlling Deposit Characteristics
Time and solution turnover control thickness. Controlling bath chemistry and stabilizer levels influences grain structure and adhesion.
Post-Plating Treatments
Rinse in deionized water after plating. You can also dry or slightly bake the part to increase adhesion. Extend silver’s brightness and life by applying anti tarnish coatings like triazole.
Immersion (Displacement) Silver Plating
Basic Principle
The silver ions reduce and deposit as a thin silver layer when you dip the metal into a silver salt bath. It is a common reaction.
Cu(s) + 2 Ag⁺(aq) → Cu²⁺(aq) + 2 Ag(s).
Electricity isn’t required here; the metal reactivity takes care of the reaction for you.
Process Characteristics
It creates a very thin silver coating, typically less than 0.1 µm thick. Immersion plating works well if you just need a light, decorative layer. It is self limiting, so the reaction slows or stops when the base metal is covered or consumed. If you prepare the surface right, you won’t get a thick layer but you will get even coverage.
Bath Composition
Generally, you will use a silver nitrate or a silver acetate solution. You may add complexing agents like ammonia to control the availability of silver ions. The solution is often mildly acidic—anticipate a pH somewhere in the range of 3 to 5 depending on your substrate.
Examples and Limitations
This method can be used for decorative parts, antique coin restoration or test plating runs. It can be used, but you shouldn’t depend on it for functional plating. It is too thin a layer and adhesion can be weak. Full electroplating is better if you need durability or conductivity.
Substrate Pre-Treatment
The metal surface must be thoroughly cleaned before plating. All oxides or contamination are removed. Adhesion is a function of a clean surface and good metallurgical bonding since there is no external current. For poor plating results, this step is skipped. For best results, always carefully prep your surface.
Materials, Substrates, and Undercoats
Common Substrates for Silver Plating
When you work with silver plating, you work with copper and brass. These are common metals in connectors and contacts in electronics. If you are using any metal except steel or stainless steel you do not need to add a strike layer, but if you are using steel or stainless steel you must add a strike layer such as nickel, to ensure the silver bonds well. For plastics and ceramics, there must be electroless copper or palladium pre treatment to make the surface conductive. If you’re using aluminum, a zincate treatment or special silver bath for non ferrous metals should be used.
Role of Strike Layers / Undercoats
Striking layers enable you to increase adhesion and barrier protection. The copper strike works well on steel, aluminum or nickel chrome layers and it makes sure the silver bonds well. The nickel strike prevents unwanted metal diffusion, especially on steel and helps in even silver coverage. For non metallic materials being plated, use palladium activation to electrolessly plate. Prepping surfaces such as ceramics or plastics is important and this step is a crucial one.
Surface Roughness and Its Impact
Surface roughness is what you should pay attention to. Micro-anchors are created due to a slightly rough surface which increases mechanical bonding and deposit strength. For electronics, a low Ra value (less than 0.2 µm) is desired. For decorative parts you may prefer a smoother or glossier finish with an Ra closer to 0.1 µm.
Compatibility and Pre-Strip Layers.
Always strip old layers before re-plating. An acid or alkaline stripper may be needed to remove nickel or tin. This step is to expose more clean base metal to give the silver better adhesion and finish quality.
Equipment, Tools, and Facility Requirements
Plating Tanks and Racks
The tanks you need are PVC, polypropylene or stainless steel tanks that resist corrosion in silver baths. Racks and fixtures made of titanium, stainless steel or brass are used. Uniform plating requires that their design ensures even current flow across all parts.
Power Supply
Use a good DC rectifier with adjustable amp or voltage control. Low ripple percentage will keep plating quality high. Programmable power supplies are the way to go if you are using pulse or reverse plating, for instance, as you need precise control.
Filtration and Circulation system.
Use high efficiency filters to keep the bath from getting particulates and metal debris. Temperature and mix are maintained using pumps or air spargers. It ensures consistency in plating results and avoids deposit defects.
Monitoring and Analytical Instruments.
An accurate pH meter should be used to regularly check bath pH. Ionic strength is monitored with a conductivity meter. Temperature probes keep bath temperatures stable. Plating thickness may be measured with XRF or coulometry to assure product quality.
Safety and Ventilation
Capture hazardous vapors (particularly from cyanide or acids) with fume hoods. Use PPE—nitrile gloves, aprons, face shields and respirator when needed. Set up spill containment trays under tanks to protect your workspace.
Applications of Silver Plating
Electronics and Electrical Contacts
Electronics rely on silver plating. On printed circuit boards (PCBs), you’ll find it as a thin silver flash that improves solderability. Silver provides low contact resistance and corrosion resistance for connectors and terminals, making sure your devices will be able to maintain reliable connections. As it has high conductivity, silver minimizes the signal loss in RF and microwave components, important for clear communication.
Jewelry, Silverware, and Decorative Items
Silver plating is in your everyday luxury. To add a shine and make cleaning easier, cutlery and serving dishes get a silver layer. Silver plating is used in ornamental objects such as picture frames and religious artifacts, to maintain their beauty. Silver’s elegant finish benefits even consumer goods such as smartphone trims, luxury watch parts, etc.
Optical and Reflective Applications
Reflectivity is enhanced by silver plating. Silver film on glass makes mirrors reflect more than 95 percent of light. And in telescope and camera mirrors you will find ultra smooth, defect free silver layers, guaranteeing clear and precise images.
Aerospace and Automotive
Silver plating is often used to improve contact surfaces in the aerospace and automotive sectors such as relays, switches and sensor contacts. It also improves the performance of reflectors and antennas, providing the conductivity and durability you need for the best performance.
Medical and Dental Devices
The antimicrobial properties of silver make it an excellent candidate for surgical instruments and catheters, lowering the risk of infection. Silver plating is used in dental alloys to provide improved appearance and prevent tarnishing so that you can continue to have a confident smile.
Some Other Niche Uses
Chemical and pharmaceutical equipment is supported by silver plating, where corrosion resistance and cleanliness are most important. It also helps restore art by re plating the historical artifacts so they can be preserved for you and the future generations.
Advantages of Silver Plating
Electrical and Thermal Conductivity
Among all metals, silver has the highest electrical conductivity, 6.3 × 10⁷ S/m. This translates to when you have silver plating, your components are excellent conductors of electricity and heat. This translates into high efficiency and lower energy loss for high frequency circuits and power devices. You get better overall performance and faster signal transmission.
Corrosion Resistance
Silver resists oxidation better than does copper which helps protect the underlying metal from rust and corrosion. Plating a surface with silver makes it last longer in normal air conditions. Though be aware that silver can tarnish in the presence of sulfur compounds, so you might require some maintenance in harsh environments.
Solderability and Wettability
Solder bonds easily to silver plated surfaces, so your joints are more reliable. Soldering on silver takes less heat than pure silver and that means you have less chance of damaging delicate parts. You end up with smoother assembly and stronger electrical connections.
Aesthetic and Reflective Qualities.
The silver will give a bright, mirror like finish that you will love in jewelry and decoration. It also reflects about 95% of visible light which makes it ideal for optical devices where you want maximum reflectance and clarity.
Biocidal Properties
Bacteria and other microbes are killed by silver ions. Selecting silver plating for medical tools or surfaces imparts an antimicrobial layer which makes them more hygienic and safe to use.
Troubleshooting Common Issues
Poor Adhesion or Peeling
The surface must be cleaned and activated thoroughly before plating. Left behind oils or oxides will weaken adhesion. Make sure you have properly put on strike or undercoat layers which prepare the surface for strong bonding. Rack contacts should also be carefully inspected. Weak spots and peeling are caused by uneven electrical connections.
Inconsistent Thickness (Uneven Plating)
If your plating thickness varies, look at your current distribution. Such effects as ‘bread loaf’ or ‘thief on edge’ indicate the uneven current flow. Fixing this involves changing the anode to cathode distance or adding shields and auxiliary anodes. The uneven deposits can be prevented by improving bath agitation or circulation.
Surface Defects (Pitting, Roughness, Burn Marks)
Gas forming at the cathode usually leads to pitting. To control this you should decrease the current density or add brighteners. You get burn marks when your current or plating time is too high — lower your current density. If you have a rough surface, check the bath for contamination, check the additives for wrong additives and last, check the temperature.
Tarnishing During or After Plating
It tarnishes from sulfur contamination or organic impurities in the bath. Residues should be rinsed from parts immediately with high purity deionized water. Rinse, quickly dry the pieces and apply an anti tarnish inhibitor for protection.
Bath Instability (Precipitation, Fogging)
Keep the correct ratio of complexing agents so that there are no free silver ions causing precipitation. Small shifts in pH trigger unwanted reactions, so keep the pH very tightly controlled. Maintain it strictly as per routine – use stabilizers, filter regularly, replenish bath when needed.
Economic Considerations and Cost Analysis
Material Costs
Silver price trends are known for being very volatile in USD per ounce. Fluctuations in these numbers are closely monitored because they directly affect your plating budgets. Moreover, electroless plating processes require consumables such as complexing agents, brighteners and reducing agents for which you will have to incur costs. While these chemicals are necessary, they increase your overall expenses.
Operational Costs
Your plating expenses depend highly on energy consumption. It is typical to use several kilowatt-hours (kWh) of power to electroplate a kilogram of silver. You also need skilled labor to run plating lines and monitor baths. You have to factor in additional recurring costs like bath maintenance and filtration systems in order to keep things running smoothly.
Cost-Benefit Analysis
When you compare silver plating to gold, nickel or tin you want to weigh performance versus cost. Aesthetics and conductivity tend to outperform silver at a reasonable price. In applications like high frequency electronics, luxury goods or high end optics, silver plating can be a substantial return on investment (ROI).
Throughput and Batch Size
Larger batches benefit from economies of scale and the cost per part is reduced. But it demands much larger tanks and more infrastructure. Flexible runs are supported with small batch or custom plating, but have the downside of longer setup and changeover times which impacts throughput.
Financial and pricing strategies.
When quoting your plating service, include scrap recovery from silver reclamation, overhead and profit margins. Forward contracts and silver recovery programs are good ways to protect yourself from silver price swings. They also stabilize your costs and your pricing.
Conclusion
Silver plating still remains a versatile and very useful method for improving appearance, conductivity and corrosion resistance in many industries. Its benefits for electronics, jewelry, medical devices or any other application – excellent electrical and thermal conductivity, antibacterial properties and superior reflectivity – cannot be ignored. Through an understanding of the different plating methods and surface preparations you can obtain durable, quality platings for specific applications. Silver plating is still a reliable performer both for the beginner and the professional in modern manufacturing.
