What is Nickel Plating? – The Ultimate Beginner’s Guide
Nickel plating is very important in enhancing the strength, the look and the resistance to corrosion of metal components. Nickel plating can be a saving grace to any worker in the manufacturing sector, design, or repairs because it helps one avoid costly errors. Still, with improper knowledge, you can end up with bad finishes, weak bonds or damaged parts. This is why this guide is important. This article will teach you everything you need to know about nickel plating, how it works, the methods used and materials involved so you make better informed decisions and have confidence in your choices.
Table of Contents
ToggleWhat is Nickel Plating?
Nickel plating is a metal finishing process in which you use a thin coating of nickel to enhance the strength, resistance, and appearance of a surface. You may apply it to prevent the corrosion or wear of parts and provide them with a polished surface. It is done by placing nickel over metals such as steel or copper. There are two primary ways: the electroplating that involves the use of electric current and electroless plating that is based on chemical reduction. Both assist you in getting lasting high-quality surfaces that can serve many purposes.

A Brief History of Nickel Plating
The history of nickel plating is rich, and that is why you can comprehend its worth nowadays. This process commenced in 1805 when Luigi Brugnatelli tried to electroplate metals by using electric current. His contribution paved the way to innovation. By 1837, the process was refined by Golding Bird, which was more stable and feasible in industry. Later on in the year 1916, Oliver P. Watts invented the famous Watts bath- a solution that made nickel plating more uniform and popular. Technology changed and so did the methods. To-day, you have at your command superior compositions of baths and additives, and extremely effective plating equipment.
How Does Nickel Plating Process Work?
Nickel plating is the application of a thin film of nickel on a surface to give durability and aesthetic appeal. You start with degreasing and cleaning the substrate. Cover the spots you do not want to plate. This may be followed by heat treatment to enhance adhesion. Then, you etch and pickle the surface in order to eliminate oxides. Once prepped, nickel can be applied by either electroplating (with electric current) or by electroless plating (chemical reduction). Lastly, wash and dry the part. Electroplating is good on simple shapes, but electroless plating provides you with even coverage on complex geometries without electrical apparatus.

Key Benefits of Nickel Plating
Corrosion Resistance
Nickel plating protects metal surfaces against moisture, chemicals and oxidation. It is usable in rugged industrial or marine conditions. This assists in increasing the service life of vital parts.
Wear and Friction Resistance
The plated layer is abrasion resistant and minimizes contact between moving surfaces. You will find a better performance of gears, valves, and tools. It is perfect on components that are in contact with each other all the time or are subject to stress.
Increased Hardness
Nickel plating includes an outer hard shell that enhances durability. This is a very good defense, in case of mechanical wear. It fortifies the structure without changing the properties of base materials.
Electrical Conductivity
It provides superior electronics, connector and PCB conductivity. Nickel plating can be utilized where signal integrity is important. It also prevents corrosion of delicate circuits.
Aesthetic Appeal
The smooth finish is bright, mirror-reflective, and improves visual performance. It finds wide application in automotive trim, consumer goods, and hardware. Nickel plating is a good appearance provider.
In comparison with Chrome or Zinc
Nickel has a better resistance to corrosion than zinc and it provides a smoother finish compared to chrome. You get an improved cost-durability-look compromise.
Chemicals Used in Nickel Plating
Knowledge of the chemistry involved in nickel plating assists you to manage quality, final appearance, and longevity. Bath composition has direct impact on adherence of plating, appearance and resistance to corrosion. Now let’s uncover, what are the chemicals involved in each of the methods?
Electroplating
There are four main chemicals that you are likely to use in nickel electroplating:
- Nickel sulfate: It is the main source of nickel ions. It facilitates the real deposition on substrate.
- Nickel chloride: It enhances conductivity and facilitates effective anode dissolution.
- Boric acid: It maintains the pH and avoids burning at high-current regions.
- Brighteners and levelers: These help you obtain a smooth, glossy finish and better uniformity.
Electroless Plating
In electroless nickel plating, no electricity will be applied. Rather, the bath is based on chemical reactions. You’ll use:
- Sodium hypophosphite: It is a reducing agent which deposits nickel on the surface.
- Stabilizers and reducing agents: These regulate the speed of the reaction and the stability of the bath.
- Complexing agents: They retain nickel ions in solution and enhance the quality of deposition.
What Is the Best Acid for Nickel Plating?
Boric acid is the best acid used in nickel plating. It assists you in stabilizing the pH, and enhances adhesion in plating. You can pre-treat with hydrochloric acid, but not with the plating bath itself, of course. pH control is essential, as it will allow a steady deposition of nickel, superior surface finish, and a reduced number of plating defects, and achieve high-quality and reliable results.
Different Types of Nickel Plating
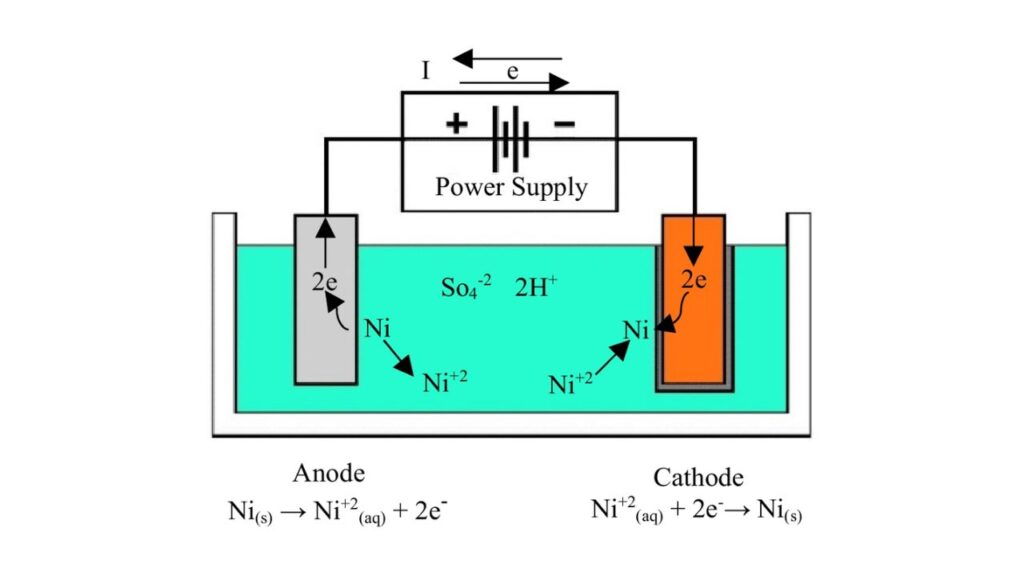
Electrolytic Nickel Plating
Electrolytic plating involves the electric current in depositing nickel on a metal surface. You place the part in a solution and pass voltage to cause the nickel build-up.
- Applications: Automotive parts, tools and decorative parts.
- Pros: You obtain a smooth and bright finish with a comparatively low price.
- Cons: The coating can be uneven on complex shapes. Some parts may require relocation to obtain good coverage.
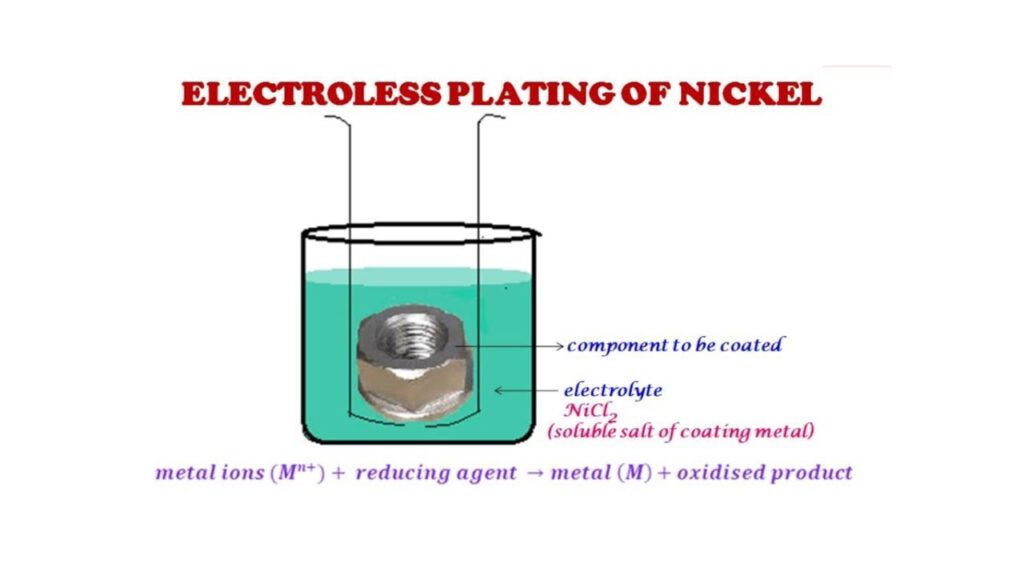
Electroless Nickel Plating
It is a chemical process, no electricity is used. The reducing agent in the bath gives the nickel a uniform deposition on the surface.
- Applications: Aerospace parts, valves, molds and electronics.
- Benefits: You achieve even coverage, even on complex shapes.
- Disadvantages: It is more costly and requires stringent control in terms of bath to sustain quality.
Bright Nickel Plating
Bright nickel plating employs brighteners which are additives that produce a high-gloss, mirror-like finish. It is widely applied in places appearance is important.
- Uses: Domestic fittings, appliances, and car trims.
- Pros: It will make your products look beautiful and ornamental.
- Disadvantages: Must be balanced chemically. Lack of control may cause brittleness or flaking.

Dull Nickel Plating
This is a non-reflective, non-brightened finish. This could be what you want, unless you are worried about appearance.
- Uses: Structural components, tools, and industrial machinery.
- Pros: It has an improved thickness and mechanical strength.
- De-merits: Ugly to look at- more functional than decorative.

Black Nickel Plating
Black nickel alloys nickel with elements such as zinc or copper, to produce a dark finish, which is frequently shiny. It is primarily cosmetic.
- Uses: Lenses, clothing accessories, circuit housings.
- Pros: Provides a contemporary, slim appearance and moderate security.
- Disadvantages: The coating is prone to faster wear than other types. It might require a topcoat to make it durable.

Nickel Sulfamate Plating
It involves nickel sulfamate to form a pure, thick and ductile layer. You will employ it in the high-stress or high-performance sections frequently.
- Applications: Aerospace components, precision tooling, post machining work.
- Pros: Forms soft and thick film with high adhesion.
- Disadvantages: It needs close supervision of the bath and handling.

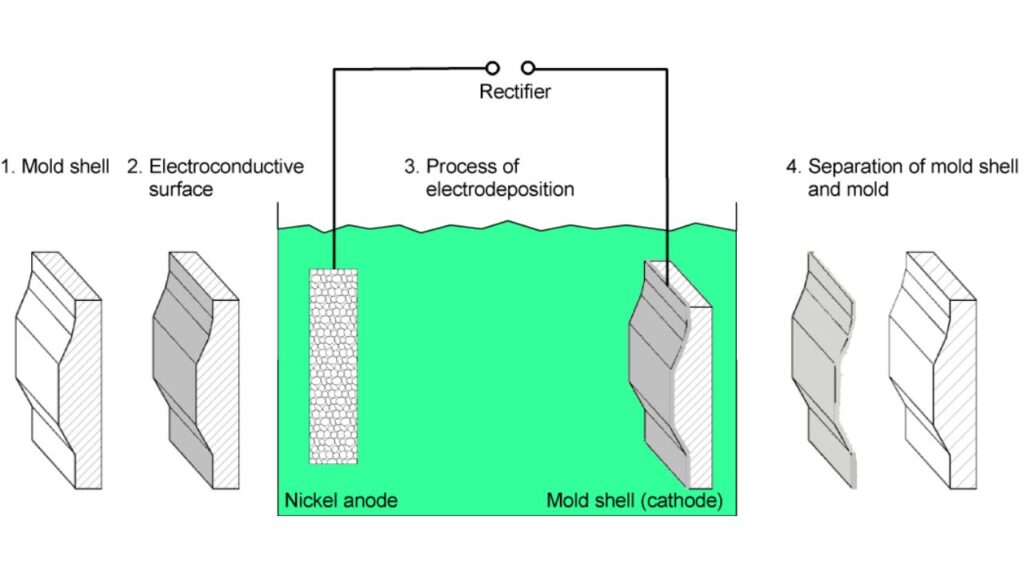
Electroforming
Electroforming grows whole components using nickel by plating over a mold, after which is removed. It is more precise in applications.
- Applications: Micro-electronics, optics, aerospace parts.
- Benefits: Good when you require very fine detail or when you require thin walls.
- Disadvantages: It is time-consuming and normally specialized.
Choosing Between Electroplating and Electroless Nickel Plating
| Feature | Electroplating | Electroless Plating |
| Method | Electrical current | Chemical reduction |
| Finish Control | Adjustable | Uniform on complex shapes |
| Cost | Lower for high volume | Higher due to chemicals |
| Coverage | Uneven on complex parts | Even and precise |
| Equipment | Power supply needed | Requires chemical monitoring |
- Use cases for each method.
- Factors to consider: part geometry, budget, aesthetic, performance needs.
What Materials Can Be Nickel Plated?
Steel
Steel is easily nickel plated. Thoroughly clean the surface to remove the oils and oxides. Acid pickling should be done to guarantee good bonding.
Copper and Alloys
Copper and its alloys such as bronze plate well. Tarnish and oxides must be removed prior to plating. Surface should be activated using mild acid dips.
Brass
Brass is a proper material, too. You will however need to remove any lead impurities and use a copper flash layer where necessary to avoid poor adhesion.
Aluminum (pre-treated)
Aluminum is fragile. It does not combine favorably with nickel. Before nickel plating, you need to carry out a zincate treatment or intermediate intermediate layer such as copper.
Zinc and Zinc Alloys
Zinc and zinc alloys are highly reactive, so it is possible to plate them. Avoid poor adhesion and blistering by first using a cyanide or alkaline copper strike first.
Cast Iron
The cast iron has a graphite that may interfere in plating. Surface impurities are removed through acid cleaning and copper strike layer applied then nickel plating.
Plastics (through electroless plating)
Plastics may be nickel plated, but only by electroless processes. Plating requires that the surface be etched, activated (usually with palladium), and sensitised, before plating.
Can You Nickel Plate Non-Metals?
Plastics
Yes, it is possible to nickel plate non-metals and the process varies depending on the material. Electroless nickel plating is the best method to use in plastics. You use it when you want some decorative or functional metallic finish, say in automotive trim or an electronics housing. You etch the plastic surface, catalyze it, and put the nickel layer on chemically, again without electricity.
Glass
Conventional nickel plating will not work, particularly when you are working on glass. Glass is non-conductive and incompatible with surface. Rather, you will require sophisticated options such as vacuum deposition or sputtering. The processes are used on controlled environments and apply a thin nickel layer, but specialized equipment is required. Therefore, even though non-metal plating can occur, it is up to you to select the appropriate technique depending on your material and intended use.
Equipment Required for Nickel Plating
Plating Tanks
The plating solution should be contained in non-reactive tanks, which are usually polypropylene or PVC tanks. These tanks have to be chemically resistant and be temperature stable.
DC Power Supply (Electroplating)
You need a good DC power supply in case you are electroplating. It assists in regulating current density, which has a direct influence on coating thickness and evenness.
Heating Units
You will need heaters to keep bath temperature ideal. Consistent plating quality and reaction rates are maintained by stable heat.
Agitators
The agitation enhances evenness by avoiding rapid depletion of the ions around the workpiece. The agitation system can be air, mechanical or solution.
Anode/Cathode Systems
High-purity nickel anodes and cathode fixtures should be used to make appropriate current flow and deposition.
Fume Hoods and Safety Gear
Wear gloves, goggles, aprons, install fume hoods all the time to guard yourself against deadly vapors.
Optional: Automated Lines
Automated plating lines could be used to save some time and increase consistency in high-volume jobs.
Key Parameters in Nickel Plating
- Bath Composition: Keep the correct ratios of the chemicals used and renew frequently preventing variations.
- Current Density: Set it to regulate the thickness and uniformity of the nickel layer.
- Temperature: Should be watched carefully since it affects the speed of reaction and the properties of adhesion.
- pH Levels: Maintain the bath in balance; unbalances may result in bad deposits.
- Agitation: Stir the solution to foster even plating on the surface.
- Deposition Time: Increase or decrease depending on the desired thickness of coating.
Chemical Nickel Plating Solution Composition
In case you are intending to perform nickel plating, it is important to know the chemical composition of the plating solution. This balance will have a direct effect on the finish, adhesion and the general quality of your coating. You will work with three major kinds of nickel plating baths, ones called Watts bath, electroless nickel bath, and sulfamate nickel bath. They all have different purposes and have distinct components.
Watts Bath (Bright Nickel Plating)
This is among the most commonly employed solutions of decorative and partial functional plating. The principal source of nickel ions will usually be nickel sulfate (NiSO 4). Nickel chloride (NiCl2) enhances the performance of the anode and the conductivity of the process, stabilizing the process. The pH is buffered by boric acid (H3BO3) and the deposit maintained to be uniform. You can also add levelers, wetting agents, and brighteners, in case you desire a bright finish.
Electroless Nickel Plating Bath
This solution does not use external electrical current. Rather, it depends on a chemical reaction between nickel salts (typically nickel sulfate or nickel acetate) and a reducing agent, such as sodium hypophosphite. You would also require stabilizers and complexing agents to maintain the process in control. It is awesome when you have to paint on complicated surfaces or non-metallic materials requiring smooth coverage.
Sulfamate Nickel Bath
Sulfamate baths are your best choice when you are dealing with precision engineering applications. They provide low internal stress, high-purity nickel deposits, which enable thicker, more ductile deposits. This renders them suitable in applications such as molds, dies or parts that will be machined.
Temperature Control Bath
An important parameter in the plating process is temperature. It affects the rate of plating, the quality of deposits, the adhesion and the internal stress. In the case of Watts baths, temperatures ranging between 40oC and 65oC should be maintained. Warm temperatures accelerate plating and possibly decrease brightness. Cooler temperatures cause things to take longer and provide a less jagged result. Autocatalytic reaction in electroless nickel baths maintains a temperature of 85oC 95oC. To control the temperature of the bath effectively, you may use thermostats, digital controllers or in-tank thermocouples.
Plating Bath pH
The pH must be maintained in a particular range so as to maintain a stable process and quality finish. Watts baths should have a pH of 4.0-5.0, 4.5-5.0 being ideal. The optimum pH range of electroless nickel baths is between 6.0 and 9.0. An increase in the pH tends to produce a brighter finish whereas a low pH produces a matte finish. Monitor and adjust using pH meters in real-time and add acids, such as sulfuric acid or bases, such as ammonium hydroxide.
Nickel Electroplating Voltage
With electroplating, voltage determines the rate at which the nickel ions travel and settle on your part. You will normally be working between 2 and 5 volts. It is best to remain between 2 and 4 volts to provide a smooth finish. Excessive voltage will lead to rough burnt deposits. Not enough of it, and you may end up with uneven and slow plating.
Current Density
This is the quantity of current per square area on your workpiece. It has a direct impact on the uniformity and thickness of the plating. You are to operate between 2 to 10 A/dm 2. Complex or detailed components should have lower densities and larger, flat surfaces should have higher densities. Excessive current density will cause stressed, nodular layers. Insufficiently, and you can get soft, loosely bonded coating.
Plating Bath Agitation
The agitation maintains the ions uniformly dispersed throughout the solution and does not cause a lack of ions in the vicinity of the workpiece. It aids you in obtaining a better uniform coating. You may agitate the air via diffusers or spargers, mechanical agitation via paddles or mixers, or even the workpiece itself. This makes sure that each area of the surface receives the same accessibility to the nickel ions.
Applications of Nickel Plating
- Automotive: It prevents corrosion of vehicle trim, engine components, and bare surfaces. You obtain extended part life with reduced maintenance. Nickel plating also adds beauty to automotive finish.
- Aerospace: It is applied on parts that are subjected to high heat and pressure. You gain more strength and heat resistance. This necessitates the importance of nickel plating on flight-critical parts.
- Machinery: It imparts wear resistance to the high-friction components such as tools and shafts. You minimize the downtime of equipment and increases the life of tools. The coating is mechanically resistant.
- Electronics: It enhances electrical conductivity, and it also guards against electromagnetic interferences. You will find this on PCB connectors, sensors and circuit housings. It assists in providing stable electronic performance.
- Consumer Goods: It makes items such as faucets, handles and fashion goods look sophisticated. You receive a clean finish that does not tarnish and wears. It is even applied to coins to make them last long with shine.
- CNC Machining: You can use it to create thin and uniform coating of between 0.004 mm- 0.05mm. This enhances part accuracy and resistance to corrosion. The coating blends in with close tolerances.
Common Mistakes and How to Avoid Them
The process of avoiding common nickel plating errors begins with the preparation and surveillance. Failure to clean the surfaces will result in poor adhesion. Misplaced coating is caused by incorrect pH or current density. Finish is influenced by unstable temperature and non-agitation. Neglecting bath leads to contamination. To avoid such problems:
- Make sure to clean and prepare surfaces.
- Keep track of pH, temperature, and current density.
- Automated controls to minimize human error.
- Agitate uniformly to get consistent deposition.
- Record all parameters and make periodic checks.
- Educate your staff on how to handle solutions and take care of the bath.
Cost Considerations in Nickel Plating Cost
Depending on a number of factors, nickel plating costs may differ. Electroless plating will generally be more expensive because it is more chemically complicated, whereas electrolytic plating will be more economical in large runs. The final price is also affected by the thickness and overall coverage area. When you are plating alloys that are more difficult to plate such as aluminum or plastics, add on more surface prep cost. There are additional costs added by environmental compliance, waste treatment being the most typical. You should also expect an average of between 0.10 to 0.50 dollars each square inch.
Time Required for Nickel Plating and Key Standards
The duration of nickel plating varies with the size of the part to be plated, the thickness required and plating technique. When using a thin coating, it should take approximately 30 minutes. The thicker coatings may require a few hours. Don never neglect cleaning and preparation time. You must adhere to some standards in order to maintain quality. ISO 4527 specifies electroless nickel plating requirements, such as thickness, adhesion and resistance to corrosion. In the case of electroplated coatings, one uses ASTM B689. When you deal with military-grade parts, use MIL-C-26074. These guidelines will guide you in obtaining repeatable, repeatable results and prevent frequent problems with plating such as uneven coverage or poor adhesion.
Conclusion
Nickel plating does not merely serve as a finishing option, but a functional and cosmetic improvement to metals as well as non-metals. You now know what nickel plating is, how the nickel plating process occurs and when to use electroless or chemical nickel plating. You have also discussed the thickness of plating, the cost of nickel plating, the composition of the bath and even cleaning nickel plating to obtain long-term durability. Be it a corrosion resistance improvement or a polished surface, the appropriate knowledge gives you the power to do it in an efficient, safe, and cost-effective manner. Know the latest, adhere to the main standards, and make sure that every part you plate is up to your expectations.
Frequently Asked Questions (FAQs)
Does Nickel Plating Crack?
Yes, it is possible to crack nickel plating, usually due to the overly thick coating or mechanical stress on the plated object. Poor surface preparation or wrong plating conditions normally result in cracks. The risk can be mitigated by using the appropriate thickness and doing a good prep prior to plating.
Does Nickel Plating Rust?
Nickel does not rust as iron does but corrodes with time. Should the coating be scratched, chipped or worn, the moisture and air can reach the base metal and it can corrode. To prevent them, it is advisable to check nickel-plated objects on a regular basis and preserve the protective covering.
Is Nickel Plating Toxic?
Toxic chemicals like nickel salts are used in plating process, and they are dangerous to health when handling. The completed nickel-plated product is however usually safe when rinsed and sealed appropriately. When processing the plating process, wear protective clothing and observe safety measures.
Can You Polish Nickel Plating?
It is possible to polish nickel plating to retain shine. Apply non-abrasive metal polish with a soft cloth. Never use harsh chemicals or abrasive pads, which will scratch the surface or wear it down. The finish is kept clean and bright with frequent cleaning.
Can Nickel Plating Be Repaired?
Yes, the small scratches or dull areas can be polished out. In cases of greater wear, pitting or peeling, you may require stripping the old coating and replating the item. When you come across some wear, you should take care of it before more damage occurs.
