Zinc Plating Ultimate Guide – Everything You Need to Know
Zinc plating is an important process that is used to prevent rust, wear, and environmental aggressiveness of metal components. Whether you use metal parts or not, you should learn about the process of zinc plating and its importance. Understanding the correct process allows you to increase durability, reduce maintenance and increase appearance. In this guide you will learn all there is to know about zinc plating including the simple concept and types of zinc plating, the tools, equipment and the step by step procedure. You will also get to know how to select the most appropriate method depending on your requirements.
Table of Contents
ToggleWhat Is Zinc Plating?
Zinc plating involves coating metal, most typically steel, with a thin layer of zinc to protect against corrosion. You will also hear it referred to as zinc electroplating or in some instances galvanization. Industries have gradually shifted cadmium to zinc because of the latter material being less toxic and environmentally friendly. Zinc provides safer handling than cadmium, as well as good corrosion protection. You will find it in automotive, construction, and manufacturing.

Why Is Zinc Plating Important?
Corrosion Resistance
Zinc plating preserves metal since it provides cathodic protection. Zinc corrodes preferentially when in contact with moisture or air, protecting the steel below. What you do obtain is a powerful protection against rust, useful in outdoor or marine environments.
Improved Mechanical Strength
When zinc plating, you increase the mechanical strength. It minimizes wear and tear, so your parts can endure longer in heavy-load applications. This extra durability is important when you are working in harsh conditions.
Aesthetic Appeal
Zinc plating provides your parts with a bright clean appearance. When appearance is important, such as with consumer products, you will be glad to see the visual quality. They also provide an option to add colors via chromate passivation to suit a particular application.
Improved Paint Adhesion
Zinc coating is an effective primer on paints and coating. Zinc plating will assist in holding paint on automobiles or structural steel, extending the life of the paint job. It eliminates peeling and enhances resistance to wear in the environment.
Electrical Conductivity
You will also enjoy the electrical property of zinc. It provides dependable electrical conductivity in enclosures and connections. In low-voltage systems, it lessens the contact resistance, enhancing the general performance.
Types of Zinc Plating Techniques
Electroplating (Standard Method)
The most popular zinc plating is electroplating. You use an electric current to put a thin coating of zinc on a metal piece. You start by cleaning and degreasing the surface. You then place the piece in a bath of zinc salts with electrodes leading to a power point. Current flows and the zinc ions are deposited on the surface of the part. This technique provides you with a smooth even finish. It is perfect on precision components in electronics, hardware, and tools.
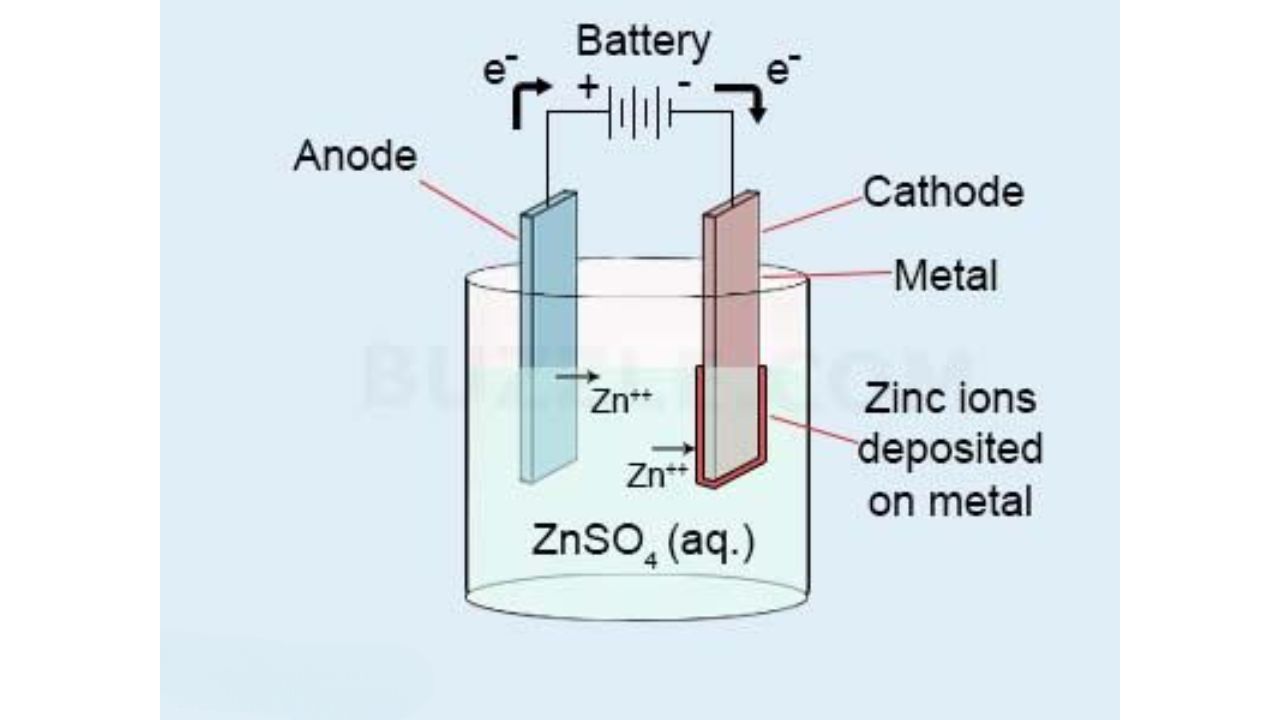
Barrel Zinc Plating Process
Barrel plating assists you in coating numerous small objects simultaneously. You put components such as screws, nuts or washers into a rotating barrel with zinc solution and electrolytes. The tumbling action provides an even coating. This process is not a high cosmetic quality method, but it is efficient and economical on high volume work. You must apply this when the appearance is not of concern yet the protection against corrosion is needed.

Rack Zinc Plating Method
Rack plating is ideal when you have fragile, large, or unusual shaped products. The parts are hung on racks and then put into the zinc solution. This is done to avoid damage and to distribute current better. It puts more control in your hands over coating thickness and the quality of the finish. Rack plating is your parts when they require a high visual appearance or have delicate areas.

Zinc-Nickel Plating Technique
Zinc-nickel plating is a process in which nickel is added to the zinc solution, usually about 12 15%. Corrosion resistance is significantly increased with this alloy. It will be commonly found in automotive and aerospace components that are subject to heat, chemicals, or salt spray. It also has good adhesion, and service life, particularly in harsh environments. Select this option when longevity is not negotiable.

Zinc-Iron Plating Process
Zinc-iron plating adds a low amount of iron to the zinc coating. It comes in handy when plating heat treated or high strength steel components. This process provides you with even coating, enhanced wear resistance, and good corrosion protection. You will find it useful on fasteners, engine components and other structural uses where strength and coating performance are important.
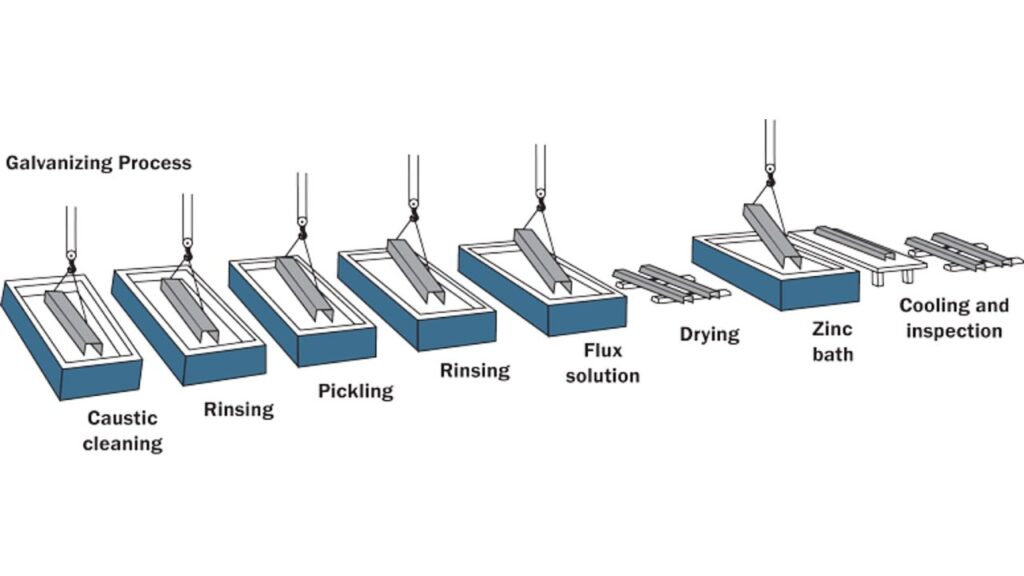
Hot-Dip Galvanizing Method
Hot-dip galvanizing is a process that entails the submergence of metal in a vat of molten zinc. The outcome is a viscous, rough coating that metallurgically binds to the base metal. You can have unsurpassed durability, yet the surface finish is often rough. Apply on heavy-scale industrial components such as guardrails, bridges, and structural beams. Remember, it is not as well suited to tight-tolerance or decorative pieces.
Zinc Chromate Coating Technique
Zinc chromate and chromate conversion coatings provide additional protection. You dip the part in chromate solution after plating. This forms a corrosion resistant film and provides color alternatives such as yellow, black or olive drab. It is extensively employed in military, aerospace, and high performance hardware both in performance and identification.

Alkaline Non-Cyanide Plating
The process is based on non-toxic, environmentally friendly alkaline solution, free of cyanide. You still achieve good adhesion and smooth finishes. It is more environmentally friendly and safer to the workers. This is the option you should consider in case you care about sustainability and safety in your shop.

Zinc Phosphate Coating Use
Zinc phosphate is not a plating coating but a conversion coating. You use it prior to painting or powder coating. It enhances paint adhesion and provides slight resistance to corrosion. You will find yourself using it a lot in automotive, primarily when preparing surfaces of body panels and frames.

Zinc Plating Process: Step-by-Step

Surface Preparation
You should clean all parts before zinc plating starts. Degrease to remove oils, then acid clean to strip rust and oxides. This is a critical step, and any contamination of the surfaces will decrease adhesion. Apply chemical cleaners, alkaline solutions and acid pickles. The cleaning performance can be enhanced by ultrasonic or mechanical agitation.
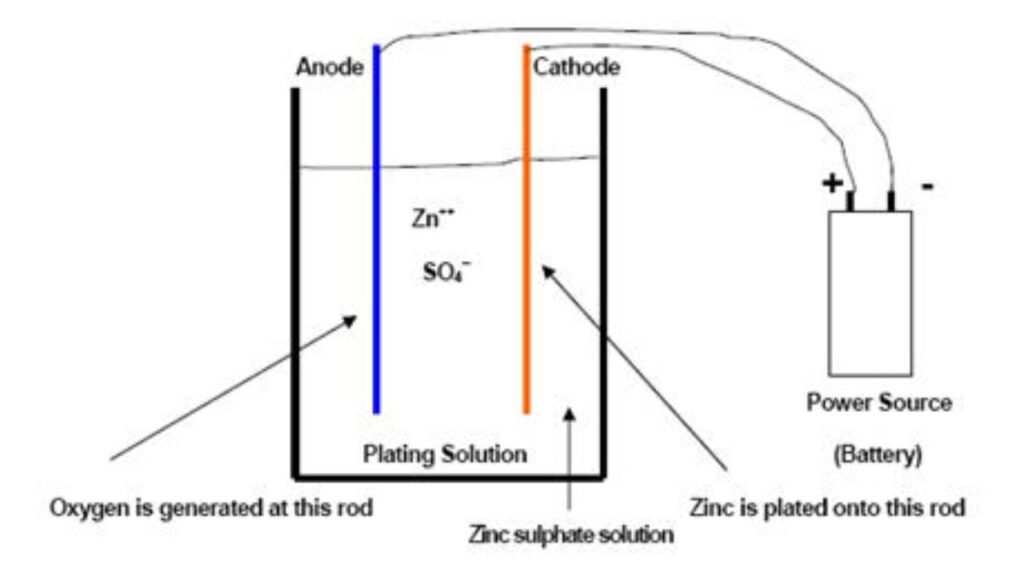
Electroplating Bath Preparation
Then you will make the plating bath with zinc salts such as zinc chloride or sulfate. Brighteners, levelers, and wetting agents are used as additives to obtain an even glossy finish. High-purity zinc is used as anode and dissolves in the electrolyte. Chemical composition will have to be balanced to ensure efficiency of the baths.
Mounting the Workpiece
Before plating, you should fix the parts well. Rack plating is most effective with large or fragile products, and provides excellent control. Barrel plating is applicable on small bulk items such as nuts or bolts. Your decision influences the uniformity of the coating, ease of handling and the plate speed.
Applying Electrical Current
Now, electroplate by applying a direct current. The movement of zinc ions is between the anode and cathode-the metal. Coating quality is directly influenced by voltage, current density, and plating time. These factors will require modification depending on the size of parts and preferred finish.
Controlling Coating Thickness
The thickness of the coating can be regulated with the help of plating time and current. Standards such as ASTM B633 or ISO 2081 provide guidance on the appropriate thickness in various environments. The thicker the coat, the more resistant to corrosion, the more expensive and time consuming.
Rinsing and Post-Treatment
Following plating, clean parts in clean water to wash residues. You will then passivate with chromate or phosphate to provide additional protection. Lastly, dry the components and look through them carefully, checking the presence of defects such as blisters, pits, or poor adhesion.
Optional Finishes
Topcoats and color chromates, such as blue, yellow, black or olive drab, can be used to increase durability. These not only increase corrosion resistance, but also assist in identification of parts or cosmetic purposes.
Materials and Equipment Used in Zinc Plating
Zinc plating can be applied to steel, iron, copper and brass. The most common are steel and iron because of the strong attachment to zinc. Copper and brass are likewise compatible but might require special surface preparation. Be sure to look at metal compatibility; otherwise, you may encounter poor adhesion or undesirable reactions during plating.
Zinc Salts
You will be using zinc chloride or zinc sulfate as the main salts in the electroplating bath. These are the source of zinc ions. To improve the performance, you can also have zinc-nickel or zinc-iron salts. These alloys have improved corrosion resistance and are suitable in severe environments.

Anodes
High-purity zinc anodes are needed in zinc plating. These will be in a shape depending on your arrangement, such as plates, spheres, or rods. Adequate anode geometry results in uniform current and coating. Change or clean them on a regular basis to uphold the quality of baths.

Electrolyte Solutions
Either acidic or alkaline electrolyte baths can be utilized. Alkaline non-cyanide alternatives are more safe and environment-friendly. They are the ones to go for when your target is worker safety and environmental compliance. Always check solution composition to get reproducible plating.
Fixtures and Handling Tools
Large or fragile parts should be plated on racks. Tumbling barrels are the best with small items. It is also possible to fit agitation systems to maintain the plating bath uniform. This prevents an uneven coating or build-up.

Control and Monitoring Equipment
Proper control is essential. Control the flow of current with rectifiers. Temperature regulators maintain constant conditions of the bath. Monitor pH and conductivity to ensure solution quality. This assists in preventing defects, and provides repeatability.
Comparison With Other Coating Methods
Zinc vs. Hot-Dip Galvanizing
Hot-dip galvanizing is thicker and more rugged than conventional zinc plating, and you will feel the difference. It is perfect outdoor or industrial. Nevertheless, zinc plating is cleaner and brighter and less expensive. When it comes to looks, use zinc. Hot-dip is superior for long-term outdoor use.
Zinc vs. Powder Coating
Zinc plating provides corrosion resistance, and powder coating provides a long-lasting decorative finish. You may mix the two to make it stronger. Powder coating is the winner when you require high color and surface durability. Look no further than zinc to have cost-effective rust protection.
Zinc vs. Anodizing
Anodize when using aluminum. Steel and iron are the best bases to zinc plate. Anodizing enhances color stability and hardness of the surface. Zinc plating increases resistance to corrosion. Select according to your base metal.
Zinc vs. Electroless Nickel Plating
Electroless nickel plating has even thickness and enhanced wear resistance. It is also more complicated and costly. Zinc plating is easier and less expensive. When accuracy and hardness are of concern, select nickel. Zinc will suffice as a general protection.
Thickness, Durability & Standards
Typical Coating Thickness Ranges
The common zinc plating thickness is between 5 µm to 25 µm. Thin coatings are used indoors and heavier ones in more severe duty. To use it heavily, exceed 12 µm to protect over time. Always align the coating with your requirement.
Plating Classifications and Finishes
Such standards as ASTM B633 and ISO 2081 assist you in selecting the appropriate finish. Appearance and corrosion requirements allow you to choose clear, black, olive, or yellow chromate. These finishes are also useful in identification of parts and functionality.
Salt Spray Test for Corrosion Resistance
You must use salt spray test to determine the resistance to corrosion. This is a simulation of severe conditions and can make you estimate field longevity. Higher rating indicates superior long term performance.
Service Life in Different Environments
Exposure is the key to the life of your plating. Indoor components have a longer life span, whereas outdoor and marine components require heavier coatings. Correct selection enhances service life.
Applications of Zinc Plating
Automotive
Zinc plating is also very common in the automotive market, used to protect fasteners, brake parts, and chassis parts. It assists these components in avoiding corrosion particularly in sections that are subjected to moisture and road salts. Through zinc coating, you enhance the life and strength of vital metal parts.
Construction
Zinc-plated screws, nail, and structural connectors in construction allow you to achieve structural integrity over the long run. These galvanized components do not rust or wear out, even when used in wet or outdoor conditions. They keep your projects safe and sound.
Electronics
Zinc plating provides electrical conductivity, so it is applicable to circuit boards and terminals. You enjoy an improved current flow and lower contact resistance. It also prevents sensitive components getting corroded, which may affect performance.
Military and Aerospace
Zinc plating qualifies under the rigorous specifications such as ASTM B633 and MIL-STD-171. You apply it to shield elements in harsh conditions. It provides even coverage and long life performance in harsh conditions.
Agriculture and Marine
Zinc plating can also protect agricultural equipment or marine equipment you may be working on so that they can last longer in water and chemicals and outdoors. It makes your equipment usable and safe in extreme environments.
Tips for Quality Control in Zinc Plating
Plating Thickness Checks
You should confirm the coating thickness in order to achieve performance requirements. Magnetic gauges when quick readings on ferrous metals are required. To be more accurate, use X-ray fluorescence (XRF). This makes the layer comply with ASTM B633 or ISO 2081.
Adhesion and Coverage Testing
Test the adhesiveness of the plating. Conduct a tape test or a bend test to identify poor adhesion. Salt spray test to ensure resistance to harsh conditions corrosion.
Surface Defect Detection
Examine components; look out for defects such as blistering, pitting, or peeling. Such defects are commonly caused by inadequate cleaning or unequal current. Early identification can assist in avoiding failure.
Routine Bath Monitoring
You should observe bath chemistry on a regular basis. Check the pH, chemical concentration and contamination. This makes the plating environment stable and the quality of the coating consistent.
Troubleshooting Common Zinc Plating Issues
Uneven Coating
You can observe uneven or spotty finishes when the agitation is poor or when the electrical current is not distributed uniformly. To remedy this, examine your rack or barrel assembly and make certain that the parts are positioned correctly. Move in a regular manner and check all connections.
Blistering or Peeling
Bubbling or flaking will be common when surfaces are not cleaned well or when the current is excessive. Be sure to clean and degrease parts. Also, maintain the voltage at recommended levels to prevent the accumulation of stress.
Low Adhesion
When the plating fails to adhere, the likely causes are oil residue or inadequate surface preparation. You ought to examine your cleaning procedure keenly. Add procedures such as alkaline soak clean, acid dipping and rinsing to guarantee bond strength.
Dull Appearance
Shine can be an indication of out-of-balance additives. Brighteners, levelers, and wetting agents should be checked and adjusted on a regular basis. To get the best results always refer to the directions given by your chemical supplier.
Hydrogen Embrittlement
You must post-bake parts following plating to avoid part failure due to trapped hydrogen. To release hydrogen safely, bake at 190230 F (88110 C) within hours of plating.
Choosing the Right Zinc Plating Method
Based on Material Type
Begin by selecting a plating technique that matches your base metal. Steel, iron, and brass are the best bases to zinc plate. When plating hardened steel, you have to use zinc-iron or zinc-nickel, which offers a better bond. With soft metals, do not use high-acid baths, which may be destructive.
Size and Shape of Components
Think about the geometry of your part. Barrel plate small, bulk pieces such as screws. Choose rack plating when the parts are larger or sensitive to get an even coating. Special fixtures may be needed on complex shapes.
Corrosion Resistance Needs
Consider exposure. When high corrosion resistance is required, zinc-nickel or chromate conversion is best. Indoors, a regular zinc electroplating may be sufficient. Align the finish with the threat.
Cost and Turnaround Time
Labor within your means. Barrel plating is quick and economical in high quantities. Rack and custom finishes are more expensive and time consuming. Schedule your plan according to your production.
End-Use Environment
Look at the environment your parts are exposed to. Outdoor or marine environments require heavier, passivated coating. A smaller layer can suffice when the application is indoor or aesthetic.
Zinc Plating Finishes and Colors
Typical Finishes
Multiple finishes of zinc plating are available: clear (blue-bright), yellow (golden), black, and olive drab. They all provide a different look and protection. A clean, bright appearance is commonly accomplished with clear finishes. Yellow offers a golden hue and moderate coverage. In military or industrial use, black and olive drab are common.
Performance of Each Finish
Every finish is not just pretty to look at. You receive the additional corrosion resistance, enhanced part identification, and industry standard compliance. Finishes also assist you in achieving particular design or performance objectives.
Typical Cost Ranges for Zinc Plating
| Application Type | Cost Range (USD) | Notes |
| Small Parts (e.g., bolts, nuts) | $0.05 – $0.25 per piece | Barrel plating is common |
| Medium Parts (e.g., brackets) | $0.30 – $1.00 per piece | Price varies by shape and finish |
| Large Parts (e.g., automotive) | $1.50 – $5.00+ per piece | Rack plating required; more labor-intensive |
| Zinc Plating by Weight | $0.30 – $0.80 per pound | Often used for bulk industrial projects |
| Custom Orders (decorative or multi-coat) | $50 – $150 per batch | Includes additional coatings or chromate |
DIY Zinc Plating: Is It Worth It?
Advantages of DIY Zinc Plating
Do you need to save money and have small metal jobs that need to be done? Do it yourself zinc plating can assist you. Basic kits allow you to restore vintage car parts, bike frames, or hand tools. There is also the fulfillment of revitalizing old metal by yourself. Simple coatings do not have to be referred to a shop.
Disadvantages of DIY Zinc Plating
Nevertheless, this procedure is associated with safety issues. Chemicals and electricity will be involved, and dangers are not imaginary. The quality of coating can be inconsistent, particularly when the right tools are not used. Thicker or industrial grade plating also gives DIY approaches trouble. You will not necessarily have a long-lasting, reliable outcome.
Suggested DIY Kits and Tools
To get started without getting shocked, use plug-and-play kits provided by reputable dealers. These usually involve plating solutions, tanks and power sources. Safety gear is not optional, wear gloves, goggles, and ventilate. Prepare a clean, dry area to work in, out of living quarters. To achieve optimal results follow instructions carefully.
Conclusion
Zinc plating is crucial in prolonging the life and aesthetic of metal parts. Whether it is the corrosion resistance or improved paint adhesion, all processes, barrel to hot-dip, address particular requirements. Knowing the correct method, apparatus and coating standard assists you in getting the best outcomes. In automotive, aerospace or general manufacturing, zinc plating enhances durability without compromising the aesthetics. This guide will provide you with the understanding to pick the most effective technique and implement it as efficiently as possible. Apply it to make your metal components more efficient and durable.
